( DITI ) Practitioner Information
For Referring Health Care Practitioners
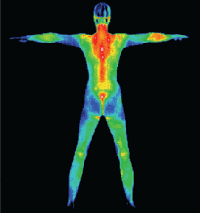
Like all medical imaging methods ……DITI is only useful within specific applications and to provide results for certain conditions and injuries.
This introductory handbook is to help all practitioners gain a better understanding of which patients would benefit from this test and how to integrate the findings into clinical evaluation, diagnosis, monitoring treatment and decision making.
DITI is a non-invasive screening technology offering clinically significant information without side effects.
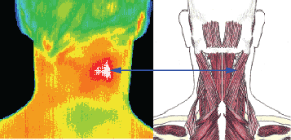
Practitioner’s are under ever increasing pressure to justify all invasive procedures.
DITI is an adjunctive diagnostic imaging technique which can provide justification for subsequent investigations or procedures.

Introduction
Thermology – is the study of human thermal physiology.
Thermography or Digital Infrared Thermal Imaging (DITI) is a non-invasive, diagnostic imaging procedure involving the detection and recording of a patient's cutaneous thermal patterns using instruments which can provide visual and quantitative documentation of these temperature measurements.
Thermography is appropriate and germane to any health care practice whenever the treating physician feels a physiological imaging test would help in a diagnosis or case management.
DITI only quantifies dermal infrared radiation (to a depth of 5.0mm +/-15%). DITI can not directly image deep structures or phenomena. However many pathophysiological states trigger thermal alterations in this 5mm of dermis via the micro-dermal circulatory beds, either intrinsically through localised phenomena, or extrinsically via effusion, vascular, neurological and/or neurovascular pathways. When used appropriately, DITI can contribute objective clinically significant data to a practitioner, allowing a greater level of confidence in diagnosis and a higher level of justification when ordering more invasive tests and procedures.
Thermal imaging has come a long way in the last twenty years, with a high level of refinement since the early 1990’s. The advances in sensitivity and reliability (of both mechanism and resultant data) are due to the huge technological advances in computing, solid state miniaturisation and declassification of military electronic super cooling and infrared technology. Today’s DITI scanners have super-cooled thermally stable receiver units capable of readings accurate to 100th of a degree.
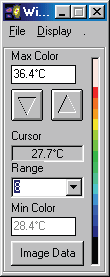
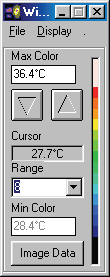
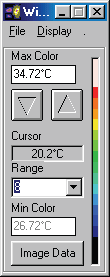
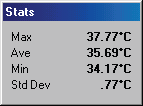
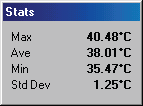
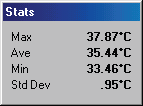
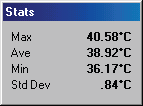
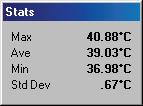
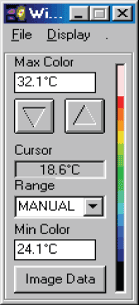
In examining a DITI image, you will often see an information box like this one.
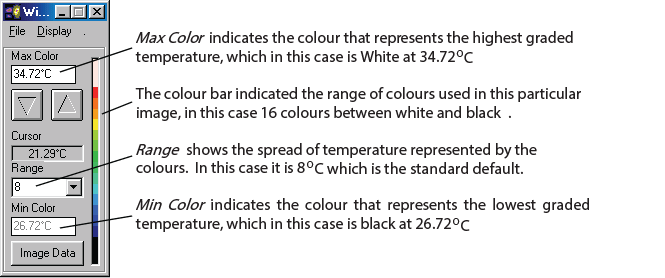
There are many varying colour maps used in DITI images. By having this colour bar and max. and min. information, a practitioner can know exactly what the displayed colours represent. As alluded to above, the default format is in a range of eight degrees, with each grade of colour representing a half degree step, running from red to blue (medical colour map). Of course, to have any clinical significance, contra-lateral studies are always in the same scale and range. If there is no colour bar information with an image, you may safely assume that it is set in the above default, excepting the actual temperature of the scale, which is adjusted to give the best image and thermal patterns for an image. For practitioners interested in referring patients, a copy of the image manipulation software is available, and the patient’s relevant images can be emailed or copied to disk and sent to the practitioner for his or her own interest.
READING THE IMAGE
It is easy to be distracted by colour, especially the higher frequencies. The main thing that DITI shows is thermal asymmetry in contra-lateral comparison, areas of unusual hypo or hyperthermia, and combined patterns. In the following case studies, you will see how the patterns indicate very different conditions.
Essentially, the pathophysiological phenomena suitable for a DITI screening investigationcan be divided into three broad categories:
1. Inflammatory phenomena
a. Infections – post surgical and common
b. Musculo-skeletal dysfunction
c. Periosteal irritations
d. Myofascial trigger points
e. Active arthritis
f. Soft tissue injury/sports injury
2. Vasomotive phenomena
a. Radicular neuropathies
b. Reflex sympathetic dystrophy
c. Neuropathological referrals
3. Vascular phenomena
a. Angiogenesis (particularly perineoplastic growth)
b. Varices (thrombophlebitis & DVT)
c. Ischaemic phenomena
Inflammatory Phenomena
Post-Surgical Infections
Post surgical assessments are hampered by a number of factors, not the least of which is the level of subjective reporting required by a patient. Varying patient expectations and experiences are confounding factors in post surgical infection assessments. DITI allows visualisation of Thermogenic phenomena both directly, and via effusion. This is limited to soft tissue and joints, with fully contained osteomyelitis demonstrating DITI findings which are equivocal at best.
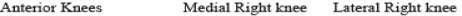

Thermogenic Infections
In cases of thermogenic infections (i.e. pyogenic infections), it is unlikely that DITI would be used in the diagnostic phase, as case history and physical inspection is normally sufficient to initiate a treatment protocol until pathology reports can return. DITI is particularly useful in quantifying the efficacy of a treatment protocol. In cases of depressed immune response or other at risk categories, where a practitioner is concerned with a protocol’s efficacy, the effusion boundaries can be clearly quantified and measured in a DITI scan. By progressive monitoring of an infection site, the regression and resolution of an active infection can be non-invasively monitored.
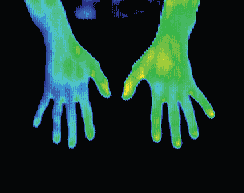
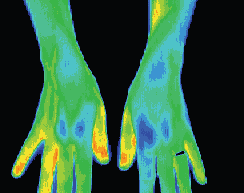
Musculo-Skeletal Dysfunction
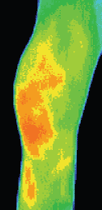
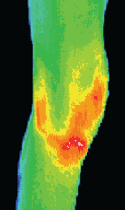
Possibly one of the more confounding patient complaints is that of musculo-skeletal dysfunction. Patient subjectivity in reporting, malingering and overlaying symptoms are just some of the confounding factors in diagnosing this group of problems. Most musculo-skeletal problems will result in inflammation of the local tissues primarily, and surrounding tissues to a lesser extent. These conditions make excellent studies in DITI terms. Because of vascular differentials in the various soft tissues, damage to muscle, tendon, ligament and periosteum all have quite distinct thermal signatures.
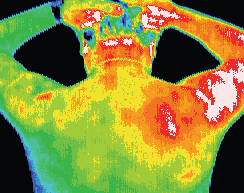
Significant inflammation throughout the right deltoid region.
Thermographic findings indicated muscular rather than joint pathology.
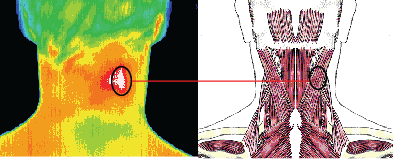
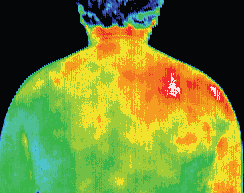
Well defined focal area of
inflammation over the splenius
capitis / splenius cervicis.
Patients headache resolved
after local IM anesthesia.
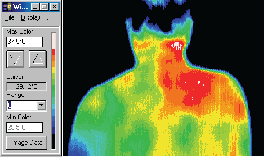
This patient with a torticollis was seen to have significant inflammation in the neck and shoulder consistent with muscle
spasm. There were significant thermal asymmetries seen
relating to the accessory nerve or the glands in the neck.
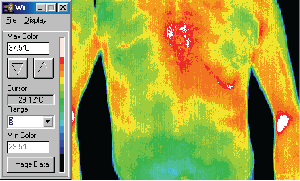
MVA patient with language difficulties, hospitalized,
examined and discharged, presented
two days later with chest pain.
Thermology findings were sent with patient
to radiology. Three fractures found in distal
sternum plus small fracture in left rib.
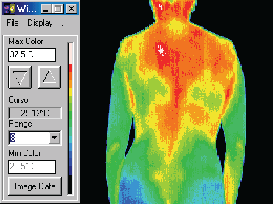
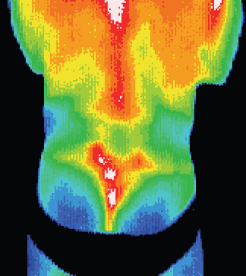
Patient with scoliosis suffering from tension headaches.
Inflammation seen over the left rhomboids was treated
with remedial massage which gave relief.
Periosteal Irritation
Stress fractures and periosteal micro-avulsion are perplexing problems for many practitioners concerned with radiation exposure levels of their patients. The most conclusive form of imaging for this type of injury is radio-isotope scanning (scintigraphy), which carries well documented radiation hazards.
DITI has a very high negative prediction rate with regard to stress fractures. By quantifying the presence or absence of periosteal irritation, which has a clear thermal signature, a positive DITI scan can justify a practitioner’s ordering of a more invasive test. A number of practitioners who are satisfied with DITI’s efficacy have begun to use just historical markers, and the DITI scans to form a diagnosis and recommend a treatment protocol.
DITI is especially useful in early cases where plain radiography often demonstrates equivocal or false negative findings,
or in young patients undergoing puberty, where radiation should be kept as low as practicable.
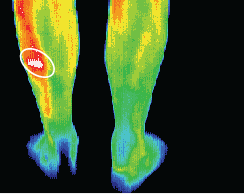
Stress fractures — not identified on X-ray but confirmed with Scintigraphy after localization with thermography.
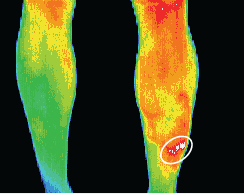
Myofascial Trigger Points
True trigger points which are active have a thermal signature that is rarely seen in any other phenomena, and is easily distinguished by historical markers. A patient complaining of a pain pattern which is following a known myofascial referral zone is a prime candidate for DITI investigation. Once a trigger point is located and quantified, the practitioner can address the problem via their preferred protocols. Interestingly, when a trigger point is seen, it is common to also see a diffuse hyperthermic pattern marking the referral zone, reinforcing the correct identification of a problem.
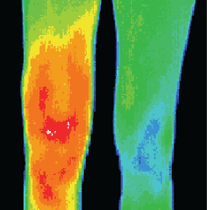
Active Arthritis
Arthritic conditions, once quantified by conventional means, can often mask other complaints. The challenge for the practitioner is one of determining whether the patient’s subjective reports are likely to be of the arthritis, or another problem in the same zone.
Active arthritis shown clearly in a DITI scan as a distinct focal area of inflammation. If there is a complaint overlying an arthritic zone, DITI has a good chance of differentiating localised joint pain, sympathetic irritation (described later in the text) and other, more wide spread inflammation indicative of a separate infection or condition.
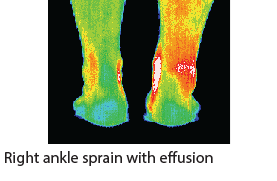
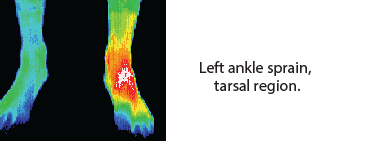
Soft Tissue Injury & Sports Injuries
Soft tissues of the musculo-skeletal system (muscle, tendon, ligament and periosteum) have very different characteristics of vascularisation and innervation. These properties allow DITI to quantify the type of tissue injured with a high level of reliability.
Damage to highly vascular tissues like muscles will typically return a DITI scan demonstrating a focal area of irritation accompanied by a large effusive area primarily proximal to the injury site. Intramuscular haematoma will demonstrate as a combined involvement in the muscle belly and associated tendinous sheath. Extra-muscular haematoma generally exhibit enough physical signs that little doubt exists as to the nature of the injury and a DITI scan will be of little value.
Tendons, being relatively avascular and aneural exhibit very different DITI signs to muscular damage. Tendinous injury will exhibit comparatively little DITI focal area, and typically a diffuse hyperthermic response, with a proximal bias.
Ligamentous injury exhibits a similar pattern to periosteal irritation. Ligament lesions tend to have a very localised inflammatory response, an immediate distal radicular neuropathic hypothermia pattern, and a proximal striated hyperthermic pattern.
Traumatic vascular injury is more effectively imaged by doppler ultrasound, and as such is typically outside of DITI’s expected examination frame.
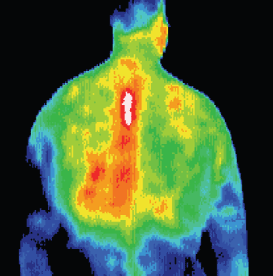
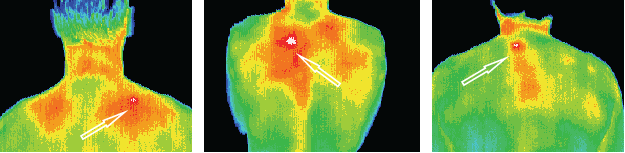
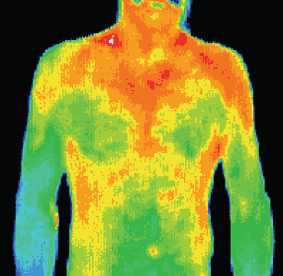
Weight lifter with a diagnosed T4 syndrome.
Thermography increased the confidence in
the diagnosis and established a baseline for
comparative studies monitoring response to
treatment.
Competitive Swimmer
with pain in left
gluteus maximus
(swimmers nemesis)
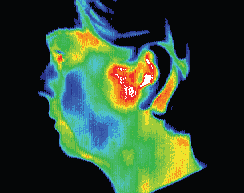
TMJ syndrome; thermography
confirming diagnosis.
 Right arm thrower with right arm weakness
Right arm thrower with right arm weakness
and paranesthesia.
Thermography showed an area of
increased motor tone (sympathetic
activity) in lower right arm with
significant temperature differentials
(hypothermia).
There is also a local area of hyperthermia
over the right brachial plexus.
Patient was treated for brachial plexus
entrapment and lower arm symptoms
resolved.
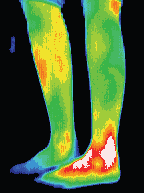 Post fracture, poor
Post fracture, poor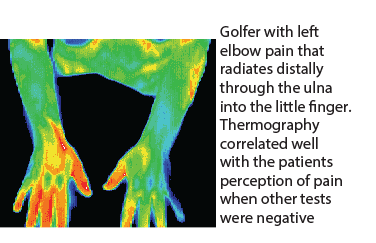
healing response
left ankle after
cast removal, being
monitored by
thermography to
help in decision
making
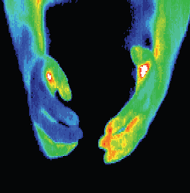
Vasomotive Phenomena
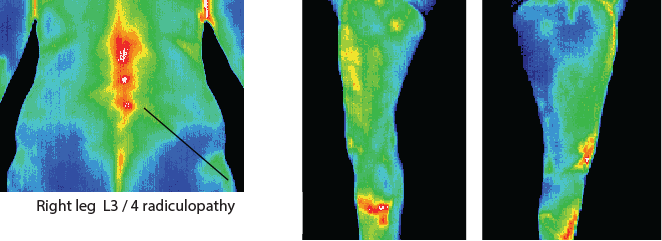
Radicular Neuropathies
Radicular neuropathies are clearly visualised by DITI scanning as an area of distinct unilateral hypothermia following a discrete sympathetic vaso-constrictive nerve supply area.
Symptoms of a radicular neuropathy may include posture dependent or independent neuralgia, anaesthesia, dysaesthesia, paraesthesia, and stiffness. As these symptoms also
cover a multitude of other aetiologies, a DITI scan is a simple, non-invasive and objective modality to quantify radicular sympathetic involvement.
Reflex Sympathetic Dystrophy (Complex Regional Pain)
The very definition of RSD is still controversial in some quarters. Many practitioners question the diagnosis of the condition due to lack of objective and reliable evidence. Hooshmand (1993) in his encyclopaedic work, “Chronic Pain: Reflex Sympathetic Dystrophy. Prevention and Management”, states that ‘Infrared Thermography is the most sensitive test in the diagnosis of RSD’. He goes on to state that no other testing modality can match thermography in the detection of RSD.
RSD has a very distinct DITI signature, where there is a well defined border to the hypothermic zone, which will normally give a ‘glove’ or “sock” like image of hypothermia. In cases where RSD is suspected, a cold challenge stress test is applied.
The suspect area is scanned for thermal stability, and once established a cold challenge is applied. The patient has a non-affected body part immersed in water of approx. 4 degrees Centigrade. This causes the sympathetic nervous system to respond, and restrict the micro-dermal circulation over the body. This sympathetic vasoconstriction will not be observed in an area demonstrating true RSD. If the symptomatic area shows any reduction in temperature over the three minutes of the cold challenge, RSD is determined not to be affecting the area. This tends towards the idea of a “simple” neuropathy. The reliability of the cold stress is guaranteed by the simultaneous scanning of asymptomatic zones, and ensuring that these zones respond with a reduced vaso-motion response.
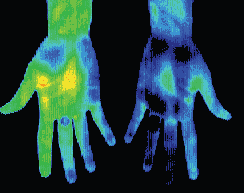
RSD patient with a ‘glove like’ hypothermia of the left hand. A temperature differential of 1.5 °c is considered significant, this patient presented with a 5 °c asymmetry. Cold stress test showed sympathetic function in the right hand but non in the left.
After treatment thermography showed good thermal symmetry and cold stress showed sympathetic function in both hands.
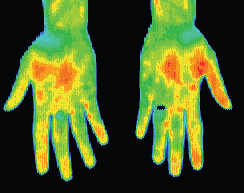
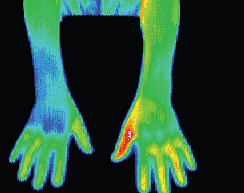
Complex Regional Pain Syndrome right foot, significant increase in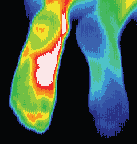 sympathetic motor tone right foot 3.7°c colder than left foot. A cold stress test was positive, (no sympathetic change). CRPS developed in the right foot after a fractured calcaneum 18 months previously. Weight bearing was painful. The diagnosis of CRPS was missed initially since nuclear imaging was not typical of CRPS. Some cases of CRPS are misdiagnosed as psychological or hysterical pain states. Thermography is able to show characteristic changes if utilized.
sympathetic motor tone right foot 3.7°c colder than left foot. A cold stress test was positive, (no sympathetic change). CRPS developed in the right foot after a fractured calcaneum 18 months previously. Weight bearing was painful. The diagnosis of CRPS was missed initially since nuclear imaging was not typical of CRPS. Some cases of CRPS are misdiagnosed as psychological or hysterical pain states. Thermography is able to show characteristic changes if utilized.
Neuropathological Referrals
Neuropathological factors in clinical assessments are made difficult by the requirement for subjective patient description of their sensations. Because DITI yields objective results, reliance on a patient’s subjective reporting is far lessened, and the practitioner is able to better understand and scale the reported symptoms.
Factors capable of interfering with the transmission of action potential will show an asymmetrical thermal signature along the path of that nerve. The neural vasomotion factors will typically exhibit alteration along a specific thermatomal pathway in the case of vertebro-costal problems, and in the specific regions of supply for problems interfering with discrete nerve pathways.
Vascular Phenomena
Angiogenesis
Neoplastic alterations of cells have demonstrated accompanying high levels of interstitial angiogenic stimulating compounds, particularly of the prostaglandin types (PEG1 & PEG2). Gullino (1992) states ‘The thin walled vessels that are present in tumours consist almost entirely of basement membrane with a single cell layer, i.e. endothelium.’ He goes on further to state, ‘the newly formed vessels produce a chaotic, disorganised network, with tortuous vessels, often sinusoidal in nature and always thin walled, traversing the tumour mass. It seems likely that the rare vessels seen in a tumour with a well-developed multi-layered wall are those that have been parasitised and engulfed by the expanding tumour mass.’ (pp. 160-161)
In suitable tissues (the breast in particular), these factors combine to exhibit a clear thermal signature due to:
• Increases in vessel size servicing the neoplasm form a demonstrable DITI asymmetry.
• The chaotic nature of the capillary structure leads to a lack of graduated thermal transition (‘smooth’ thermal signature), as expected in normal tissues.
• The lack of smooth muscle tissue in the neovascularity demonstrates a comparative vasomotion thermal signature that does not respond in similar fashion to the surrounding ‘normal’ vascular structures. Eliciting a sympathetic constrictive response by immersion of a body part (usually a hand and wrist) in cold water will demonstrate a vasomotion differential in the effected area.
• In more advanced neoplastic formations, the chaotic tissue structure with depressed metabolism in tissues removed from the vascular supply will often demonstrate a hypothermic core. Depending on position, the hypothermic displacement will show in a DITI scan.
Many of the so-called ‘false positives’ of DITI breast screening are often ‘true positive’ findings of angiogenesis preceding actual tumour development. Detection in these early stages is unreliable by conventional means, often due to the fact that the tumour has not developed sufficient density.
DITI breast screening is particularly useful to:
• Women who are under 40
• Women who for any reason can not or will not have a mammogram
• Women who are outside of the mammographic screening guidelines due to surgical procedures or contra indicating reasons.
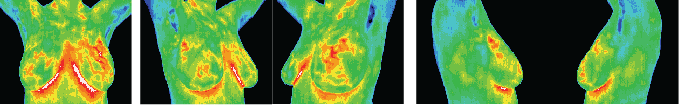
Standard views for a breast study: vascular patterns in the upper quadrants of the left breast are consistent with angiogenesis and would justify further investigation and regular comparative thermography to monitor changes.
Positive comparative study showing changes over one year:
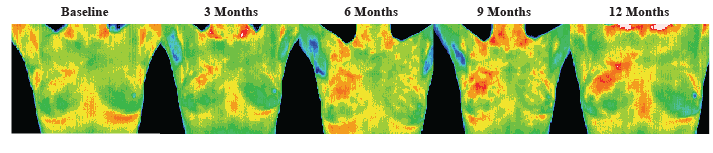
This patient was also age 37 when her first baseline thermogram showed a slight hyperthermic asymmetry in the upper right breast. The follow-up study showed the pattern had become more well defined and although clinical correlation did not find anything remarkable it was decided to repeat the exam again in a further 3 months, when again significant changes were seen. Mammography was performed at this stage with the thermographic guidance of the locally suspicious area at 1 O’clock to the right nipple. The mammographic findings were inconclusive and the patient was referred for a repeat mammogram in 12 months. Thermographic monitoring was continued and at the fifth comparative study at 12 months significant changes were still evident and the hyperthermic asymmetry (temperature differentials) had increased. Immediate further investigation was strongly recommended despite a scheduled mammogram in 6 months, and at the patients insistence a repeat mammogram was performed which clearly showed a small calcification (1 mm) at 1 o’clock. Within one week a lumpectomy had been performed with good margins and the pathology confirmed as a malignant carcinoma (DCIS).
This patient has now had stable thermograms for the last 2 years and is expected to remain healthy.

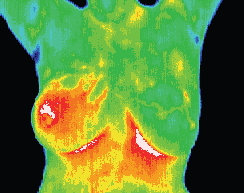
Inflammatory Breast Disease
The results of this routine study led to the diagnosis of inflammatory
carcinoma in the right breast. There were no clinical indications
at this stage. (Thermography can show significant indicators
several months before any of the clinical signs of inflammatory
breast disease, skin discoloration, swelling and pain). Inflammatory
breast disease cannot be detected by mammography and is
most commonly seen in younger women, the prognosis is always
poor. Early detection provides the best hope of survival.
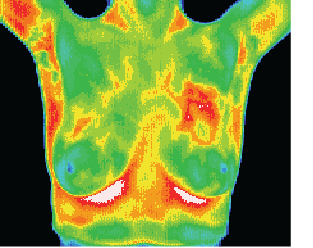
DCIS with accompanying angiogenesis
This 37 year old patient presented for routine thermographic
breast screening, she was not in a high risk category and had
no family history. No breast exams had been performed
previously. The vascular asymmetry in the upper left breast
and the local hypothermia at 11 O’clock was particularly
suspicious and subsequent clinical investigation indicated a
palpable mass at the position indicated. A biopsy was
performed and a DCIS of 2 cm was diagnosed.
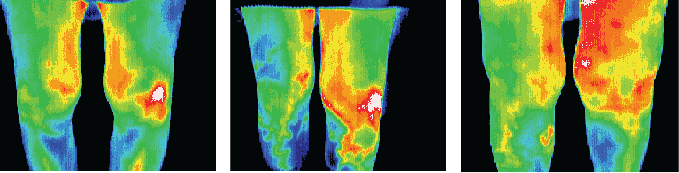
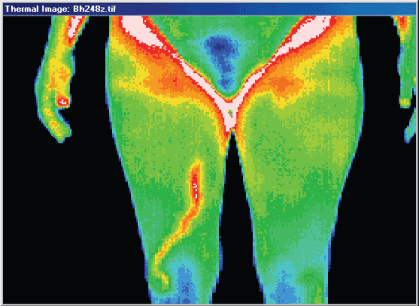
Thrombophlebitis & DVT
Thrombophlebitis is a common cause of pain in situations of varicose veins, especially in deep phenomena. Painful legs are one of the most common presenting complaints in this
condition. Practitioners suspecting phlebitis can refer for a simple DITI scan to confirm or rule out the presence of varicosities.
Increase in venous diameter results in increased volume, decreased serous velocity and often inflammation. The increase in comparatively warmer blood (volume) contrasts with the surrounding ‘normal’ tissues with typical vascular patterns. The sympathetic reflection of the inflammatory process, and the increase in relative temperature stands out spectacularly in a DITI scan.
DVT is a concern for all practitioners. The symptoms of DVT are equivocal in many cases, and best practice often requires caution to the point of excess. Pharmaceuticalagents are often
prescribed prior to definitive imaging evidence of the presence of DVT, typically by Doppler ultrasound.
DITI is capable of clearly visualising an enlarged and inflamed vein. A patient with a symptomatic presentation supporting the suspicion of DVT can have an economical DITI
scan, and if the scan is returned as positive for a varicosity, then a far more expensive Doppler ultrasound is justified. This good visualisation capability of varices gives DITI a very high level of negative prediction screening reliability.
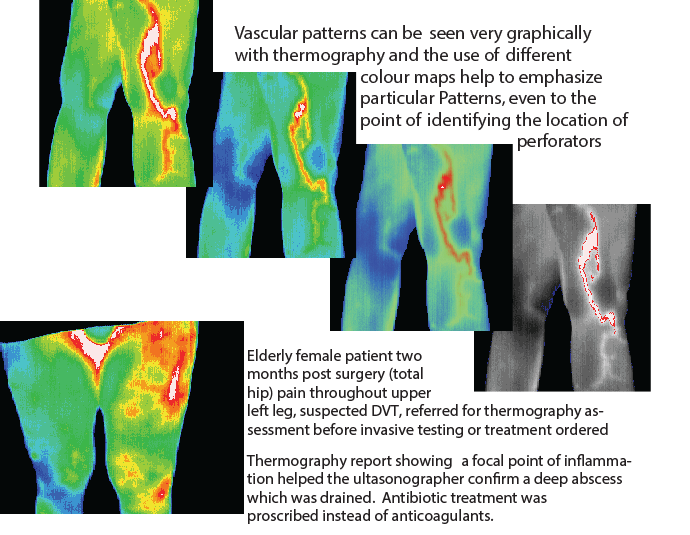
Normally the temperature of the two legs is equal at the same level, with a gradual cooling of 4°C from the inguinal region to the feet.
If there is a homogenous area of increased temperature in the calf together with loss of pretibial coolness this indicates a thrombosis in the calf veins.
Positive Thermogram scan shows deep
varicosity – unable to be seen on skin’s surface
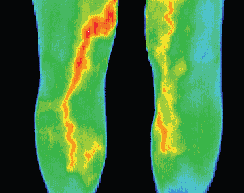
 Outpatients commonly present
Outpatients commonly present
with pain and/or swelling
of the lower extremity.
Inpatients may offer similar
complaints, but more often
the attending physician is
alerted by swelling, warmth,
redness, or venous engorgement
of the leg or thigh.
When risk factor knowledge
and examination findings lead
the physician to be
"suspicious" for DVT, he/she
must then decide between
alternative courses.
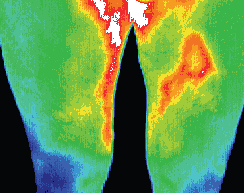
Loss of prepatellar coolness implies a thrombosis in the popliteal vein and a diffuse rise in the thigh indicates a femoral vein thrombus.
Loss of the normal temperature gradient indicates probable bilateral deep vein thrombosis.
Ischaemic Phenomena
Conditions in which arterial blood flow is reduced can typically be seen by DITI scanning. Disruptions to appendicular vessels in particular are easily visualised with DITI scanning in most cases.
A rule out ischaemia DITI scan is performed in three phases, generally taking around forty-five minutes. The first part involves imaging the symptomatic area, and its contralateral partner, as well as the vertebral innervation and primary arterial supply of the area in question.
The next part of the test involves having the patient duplicate activity that typically causes the symptoms to manifest (walk on a treadmill in the case of a femoral artery). Images of the problematic area whilst the symptoms are manifesting are captured. The last phase consists of imaging the area fifteen minutes after cessation of activity. Scan comparison gives a strong opportunity to support the theory of the pain being of ischaemic or neural aetiology. In situations where a patient’s complaint is suggestive of an ischaemic problem, a DITI scan can quickly contribute solid objective data towards a practitioner’s diagnosis.
References.
Altchek EM; Medical thermography and its use in posttraumatic cephalagia. (Int. Neurosci, 1990 Sep)
Baglin TP; Bone marrow hypervascularity in patients with myelofibrosis identified by infra-red thermography. (Clin LabHaematol, 1991)
Ben-Eliyahu DJ; Infrared thermographic imaging in the detection of sympathetic dysfunction in patients with patellofemoralpain syndrome [published erratum appears in J Manipulative Physiol Ther 1992 Jul-Aug;15(6):precedingtable of contents] (J Manipulative Physiol Ther, 1992 Mar-Apr)
Birdi N; Childhood linear scleroderma: a possible role of thermography for evaluation. (J Rheumatol, 1992 Jun)
Bruehl S; Validation of thermography in the diagnosis of reflex sympathetic dystrophy. (Clin J Pain, 1996 Dec)
Canavan D; Electronic thermography for the assessment of mild and moderate temporomandibular joint dysfunction.(Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1995 Jun)
Chan EK; Visualization of dynamic subcutaneous vasomotor response by computer-assisted thermography. (IEEETrans Biomed Eng, 1990 Aug)
Chan FH; Generation of three-dimensional medical thermograms. (Biomed Mater Eng, 1996)
Chan FH; Thyroid diagnosis by thermogram sequence analysis. (Biomed Mater Eng, 1995)
Cline M, Ochoa J, Torebjork E; Chronic hyperalgesia and skin warming caused by sensitized c nociceptors. (Brain,1989)
Cole RP; Thermographic assessment of hand burns. (Burns, 1990 Feb)
Cooke ED; Reflex sympathetic dystrophy and repetitive strain injury: temperature and microcirculatory changes followingmild cold stress. (J R Soc Med, 1993 Dec)
Dalla Volta G; The disappearance of the "cold patch" in recovered migraine patients: thermographic findings(Headache, 1991 May)
Darton K; The use of infra-red thermography in a rheumatology unit (Br J Rheumatol, 1990 Aug)
Devulder J; Epidural spinal cord stimulation does not improve microvascular blood flow in neuropathic pain.(Angiology, 1996 Dec)
Devulder J; Infra-red thermographic evaluation of spinal cord electrostimulation in patients with chronic pain afterfailed back surgery. (Br J Neurosurg, 1996 Aug)
Diakow PR; Differentiation of active and latent trigger points by thermography. (J Manipulative Physiol Ther, 1992Sep)
Dotson R, Ochoa J, Marchetti P, Cline M; Sympathetic neural outflow directly recorded in patients with primary autonomicfailure. (Neurology, 1990;40:1079-1085)
Emery RW; Revascularization using angioplasty and minimally invasive techniques documented by thermal imaging.(Ann Thorac Surg, 1996 Aug)
Feldman F, Nickoloff D.;Normal thermographic standards for the cervical spine and upper extremities. (InternationalSkeletal Society 1984)
Feldman F; Thermography of the hand and wrist: practical applications. (Hand Clin, 1991 Feb)
Friedman MS; The use of thermography in sympathetically maintained pain. (Iowa Orthop J, 1994)
Garagiola U; Telethermography and Raynaud's phenomenon. (J Sports Med Phys Fitness, 1991 Mar)
Garagiola U; Use of telethermography in the management of sports injuries. (Sports Med, 1990 Oct)
Geatti O; A comparison of scintigraphy, thermography, ultrasound and phlebography in grading of clinical varicocele.(J Nucl Med, 1991 Nov)
Graff-Radford SB; Thermographic assessment of neuropathic facial pain. (J Orofac Pain, 1995 Spring)
Gratt BM; Electronic thermography in the assessment of internal derangement of the temporomandibular joint. A pilotstudy. (Oral Surg Oral Med Oral Pathol, 1991 Mar)
Gratt BM; Future applications of electronic thermography. (J Am Dent Assoc, 1991 May)
Gratt BM; Thermographic assessment of craniomandibular disorders: diagnostic interpretation versus temperaturemeasurement analysis. (J Orofac Pain, 1994 Summer)
Gratt BM; Thermographic characterization of osteoarthrosis of the temporomandibular joint. (J Orofac Pain, 1993Fall)
Gratt BM; Thermographic characterization of the asymptomatic temporomandibular joint. (J Orofacial Pain, 1993 Winter)
Greenstein D; Assessment of chemical lumbar sympathectomy in critical limb ischaemia using thermal imaging. (Int JClin Monit Comput, 1994 Feb)
Gross EJ; Experimental assessment of phased-array heating of neck tumours. (Int J Hyperthermia, 1990 Mar-Apr)
Harper CM Jr; Utility of thermography in the diagnosis of lumbosacral radiculopathy (Neurology, 1991 Jul)
Hauer JL; Hand skin blood flow in diabetic patients with autonomic neuropathy and microangiopathy. (Diabetes Care,1991 Oct)
Head JF; Breast thermography is a noninvasive prognostic procedure that predicts tumor growth rate in breast cancerpatients. (Ann N Y Acad Sci, 1993 Nov 30)
Herrick A; Abnormal thermoregulatory responses in patients with reflex sympathetic dystrophy syndrome. (J Rheumatol,1994 Jul)
Heywang-Köbrunner SH; Nonmammographic breast imaging techniques. (Curr Opin Radiol, 1992 Oct)
Hsieh JC; Clinical application of infrared thermography in diagnosis and therapeutic assessment of vascular ischemicpain [published erratum appears in Ma Tsui Hsueh Tsa Chi 1991 Mar;29(1):567] (Ma Tsui Hsueh Tsa Chi, 1990 Dec)
Hunold S; Thermographic studies on patterns of skin temperature after exercise. (Eur J Appl Physiol, 1992)
Itoh Y; Use of recovery-enhanced thermography to localize cutaneous perforators. (Ann Plast Surg, 1995 May)
Iwata G; Thermography in a child with varicocele. (Eur J Pediatr Surg, 1992 Oct)
Janssens LA; Trigger point therapy. (Probl Vet Med, 1992 Mar)
Jeracitano D; Abnormal temperature control suggesting sympathetic dysfunction in the shoulder skin of patients withfrozen shoulder. (Br J Rheumatol, 1992 Aug)
Karstetter KW; Use of thermography for initial detection of early reflex sympathetic dystrophy. (J Am Podiatr MedAssoc, 1991 Apr)
Katoh K; Use of prostaglandin E1 (lipo-PGE1) to treat Raynaud's phenomenon associated with connective tissue disease:thermographic and subjective assessment. (J Pharm Pharmacol, 1992 May)
Kruse RA Jr; Thermographic imaging of myofascial trigger points: a follow-up study. (Arch Phys Med Rehabil, 1992Sep)
Kyle V; Rarity of synovitis in polymyalgia rheumatica (Ann Rheum Dis, 1990 Mar)
Lawson W; Infrared thermography in the detection and management of coronary artery disease. (Am J Cardiol, 1993Oct 15)
Leclaire R; Diagnostic accuracy of technologies used in low back pain assessment. Thermography, triaxial dynamometry,spinoscopy, and clinical examination. (Spine, 1996 Jun 1)
Liddington MI; Timing of the thermographic assessment of burns. (Burns, 1996 Feb)
MacDonald AG; Microwave thermography as a noninvasive assessment of disease activity in inflammatory arthritis.(Clin Rheumatol, 1994 Dec)
Magerl W; Asymmetry and time-course of cutaneous sympathetic reflex responses following sustained excitation ofchemosensitive nociceptors in humans. (J Auton Nerv Syst, 1996 Feb 5)
Mannara G; Ethyl alcohol induced skin temperature changes evaluated by thermography. Preliminary results. (BollSoc Ital Biol Sper, 1993 Oct)
Matsumura H; Haemodynamic changes in early phase reflex sympathetic dystrophy. (Scand J Plast Reconstr SurgHand Surg, 1996 Jun)
McBeth SB; Thermographic assessment of temporomandibular disorders symptomology during orthodontic treatment.(Am J Orthod Dentofacial Orthop, 1996 May)
McCulloch J; Thermography as a diagnostic aid in sciatica. (J Spinal Disord, 1993 Oct)
McKinna JA; The early diagnosis of breast cancer–a twenty-year experience at the Royal Marsden Hospital. (Eur JCancer, 1992)
Menachem A; Levator scapulae syndrome: an anatomic-clinical study. (Bull Hosp Jt Dis, 1993 Spring)
Mirza N; Influence of age on the 'nasal cycle'. (Laryngoscope, 1997 Jan)
O'Reilly D; Measurement of cold challenge responses in primary Raynaud's phenomenon and Raynaud's phenomenonassociated with systemic sclerosis. (Ann Rheum Dis, 1992 Nov)
Park ES; Comparison of sympathetic skin response and digital infrared thermographic imaging in peripheral neuropathy.(Yonsei Med J, 1994 Dec)
Pawl RP; Thermography in the diagnosis of low back pain. (Neurosurg Clin N Am, 1991 Oct)
Pierart J; Use of thermography in the differential diagnosis of phylloides tumour. (Br J Surg, 1990 Jul)
Ping Z; Correlation study on infrared thermography and nerve root signs in lumbar intervertebral disk herniation patient:a short report [published erratum appears in J Manipulative Physiol Ther 1993 Oct;16(8):560] (J Manipulative
Physiol Ther, 1993 Mar-Apr)
Plaugher G; Skin temperature assessment for neuromusculoskeletal abnormalities of the spinal column. (J Manipulative
Physiol Ther, 1992 Jul-Aug)
Raj P; Practical Management of Pain. Mosby Year Book Inc. 1992
Ramlau C; Combination of thermographic and ultrasound methods for the diagnosis of female breast cancer. (Eur J
Gynaecol Oncol, 1993)
Seifalian AM; Comparison of laser Doppler perfusion imaging, laser Doppler flowmetry, and thermographic imagingfor assessment of blood flow in human skin. (Eur J Vasc Surg, 1994 Jan)
Seppey M; Facial thermography during nasal provocation tests with histamine and allergen. (Allergy, 1993 Jul)
Sheinberg M; Application of telethermography in the evaluation of preterm premature rupture of the fetal membranes.(Biomed Instrum Technol, 1996 Nov-Dec)
Shetty V; Thermographic assessment of reversible inferior alveolar nerve deficit. (J Orofac Pain, 1994 Fall)
Sterns EE; Thermography as a predictor of prognosis in cancer of the breast. (Cancer, 1991 Mar 15)
Sterns EE; Thermography. Its relation to pathologic characteristics, vascularity, proliferation rate, and survival of patientswith invasive ductal carcinoma of the breast. (Cancer, 1996 Apr 1)
Sterns EE; Vascularity demonstrated by Doppler ultrasound and immunohistochemistry in invasive ductal carcinomaof the breast. (Breast Cancer Res Treat, 1996)
Strong WE; Does the sympathetic block outlast sensory block: a thermographic evaluation. (Pain, 1991 Aug)
Sucher BM; Thoracic outlet syndrome–a myofascial variant: Part 1. Pathology and diagnosis. (J Am OsteopathAssoc, 1990 Aug)
Takahashi Y; Thermal deficit in lumbar radiculopathy. Correlations with pain and neurologic signs and its value forassessing symptomatic severity. (Spine, 1994 Nov 1)
Tchou S; Thermographic observations in unilateral carpal tunnel syndrome: report of 61 cases. (J Hand Surg [Am],1992 Jul)
Thomas D; Computerised infrared thermography and isotopic bone scanning in tennis elbow. (Ann Rheum Dis, 1992Jan)
Thomas D; Infrared thermographic imaging, magnetic resonance imaging, CT scan and myelography in low backpain. (Br J Rheumatol, 1990 Aug)
Thomas D; Somatic sympathetic vasomotor changes documented by medical thermographic imaging during acupunctureanalgesia. (Clin Rheumatol, 1992 Mar)
Ulmer HU; Thermography in the follow-up of breast cancer patients after breast-conserving treatment by tumorectomyand radiation therapy. (Cancer, 1990 Jun 15)
Vecchio PC; Thermography of frozen shoulder and rotator cuff tendinitis. (Clin Rheumatol, 1992 Sep)
Verdugo RJ; Use and misuse of conventional electrodiagnosis, quantitative sensory testing, thermography, and nerveblocks in the evaluation of painful neuropathic syndromes. (Muscle Nerve, 1993 Oct)
Vujci M; Thermography in the detection and follow up of chondromalacia patellae. (Ann Rheum Dis, 1991 Dec)
Weinstein SA; Facial thermography, basis, protocol, and clinical value. (Cranio, 1991 Jul)
Weinstein SA; Thermophysiologic anthropometry of the face in Homo sapiens. (Cranio, 1990 Jul)
Williams KL; Thermography in screening for breast cancer. (J Epidemiol Community Health, 1990 Jun)
Winsor D; Comparison of various noninvasive techniques for evaluating deep venous thrombosis. (Angiology, 1991Oct)
Yang WJ; Literature survey on biomedical applications of thermography. (Biomed Mater Eng, 1992 Spring)
Zhang D; Clinical observations on acupuncture treatment of peripheral facial paralysis aided by infra-red thermography–a preliminary report. (J Tradit Chin Med, 1991 Jun)
Zhang D; Research on the acupuncture principles and meridian phenomena by means of infrared thermography.(Chen Tzu Yen Chiu, 1990)
INFORMATIONAL REPORT OF THE COUNCIL ON SCIENTIFIC AFFAIRS Thermography in Neurological and MusculoskeletalConditions John H. Moxley, III, M.D., Chairman